Hi everyone,
I’m culturing primary motor neurons from E13 mouse embryos and facing some issues I’d love help troubleshooting.
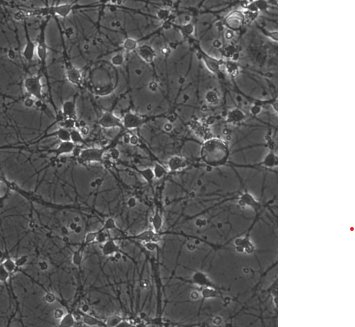
After a few days in culture, I noticed the appearance of small dot-like particles in the medium and on the neurons themselves. Initially, I thought it might be contamination, but the particles are static and consistent across wells — so it seems unlikely to be bacterial or fungal.
However, as the days go on, the neurons begin to show signs of stress:
- Loss of clear neurite structure
- Less spreading
- No detectable calcium activity (using GCaMP)
- Increased debris and non-neuronal cell death
I’ve attached an example image from the culture showing the particles and neuron condition.
Any advice on:
- Improving coating technique
- Preventing particle formation
- Optimizing motor neuron health and activity
would be greatly appreciated.
Thanks in advance!